Partial reprogramming deep dive: the good, bad, and partially unresolved
Last updated: May 4th, 2023
Right now, partial reprogramming is the hottest topic in longevity. The field went from being overlooked to becoming one of the most well-funded research areas after billions of dollars in investment into projects like NewLimit (Brian Armstrong) and Altos Labs (Yuri Milner, Jeff Bezos).
But how promising is it exactly? I decided to spend a few months learning all I could in an attempt to answer that question (see detailed notes on all the papers I’ve read here). This deep dive is a culmination of that effort, summarizing what I’ve learned about the current state-of-the-art and limitations of this field.
You can comment on this post on Twitter.
1. What is partial reprogramming?
1.1. A brief history
A history of partial reprogramming. See full list of papers here.
In 2006, Shinya Yamanaka discovered four transcription factors (Oct4, Sox2, Klf4, C-Myc), aka OSKM factors or Yamanaka factors, that can transform any cells back into stem cells. These transformed cells are called induced pluripotent stem cells (iPSCs), and the process is called reprogramming. Since the discovery, there have been several applications to create iPSCs for therapeutic purposes. In the process, people started seeing that these iPSCs gained youthful characteristics as well. Thus we know reprogramming produces two effects: 1) dedifferentiation (turning differentiated cells to stem cells), and 2) potentially rejuvenation (making cells younger).
The catch is that dedifferentiation causes cancer, but what if we could get rejuvenation without dedifferentiation? In 2010, Prim Singh proposed exactly that: instead of applying Yamanaka factors "fully" until the cell turns into a stem cell, we apply them "partially" and rejuvenate the cell without the dedifferentiating effects. That’s how the concept of partial reprogramming for rejuvenation was born.
It was not until 2016 that the theory had success in mice in vivo. Ocampo et al. were able to extend mice's median lifespan by 33%-50% by applying partial reprogramming to mice with progeria (a condition that causes mice to age more rapidly). Since then, other researchers have been trying to find the perfect cocktail combination on normal old mice and old human cells.
1.2. Terminology
Partial reprogramming comes by many names and synonyms so let's be clear about the terms:
Partial reprogramming (aka transient reprogramming): this refers to the technique of applying Yamanaka factors partially to the cells (instead of full reprogramming where cells turn into stem cells), in hope the cells may be rejuvenated without loss of their identity
Epigenetic reprogramming:
Epigenetics consists of chemical modifications to the DNA that change gene expression without changing the DNA sequence itself. Epigenetic dysregulation is considered a hallmark of aging — there has been correlative evidence that as we age, these chemical modifications start going wrong and negatively affect the cells.
Epigenetic reprograming refers to the process of changing the epigenetic landscape.
In the context of aging, epigenetic reprogramming refers to rejuvenation treatments that changes the epigenome. Therefore it includes partial reprogramming, but also includes other techniques such as parabiosis or metabolic manipulations.
Outside of aging, any process that changes the epigenetic landscape is called epigenetic reprogramming.
Rejuvenation programming: a new term invented by Altos Labs. This is a good thread on speculation of the term’s meaning. In short, the term is intended to be broad enough to capture the company’s mission without constraining themselves to particular techniques or pathways.
How different terms used to describe partial reprogramming relate to each other
1.3. How does it work?
Partial reprogramming is generally induced as follows:
First, the OSKM system is delivered into the cell or the animal. The system consists of:
OSKM, which is controlled by a promoter
The promoter, which is controlled by an activator
The activator, which is activated by doxycycline (dox), an antibiotic
The OSKM system
2. Then, dox is used to control the expression of the OSKM system. This can be done by putting dox into the drinking water of the animal or putting dox onto the cell media over a period of time. The longer you give dox to the cell or animal, the more OSKM is expressed. Thus, you can see that different studies use different duration and dosages of dox to control how much reprogramming is done (you want to do it just right so that the cell is rejuvenated without being dedifferentiated).
In the first step, how does the OSKM system get delivered? You can either deliver it to normal mice with viral vectors, or you can work with reprogrammable mice, which already have the system in their cells.
Viral vector delivery: In order to replicate and survive, viruses introduce their genetic material into host cells. We can therefore hijack this capability to deliver the genes we want by packaging the OSKM system into viral vectors then injecting them into the cells or animals. The most commonly used vectors are from lentiviruses and adeno-associated viruses.
Reprogrammable mice: Some mice were born this way, as Lady Gaga said. These mice have the OSKM system in almost every cell of their body, so all you need to do is to give them dox to induce reprogramming. How do these mice get produced? Basically, the OSKM system is delivered to mice embryonic stem cells, and these cells are injected into mice blastocysts to establish a mouse line that can produce reprogrammable mice.
How reprogrammable mice are made. Image credit.
Another less common way to induce partial reprogramming is through mRNA cocktail. Instead of using the OSKM system, you can deliver the OSKM mRNAs into the cells through an mRNA-lipid complex. In this method, you don’t control the amount of OSKM expression through dox, but through how much mRNAs you deliver into the cells.
1.4. A summary of partial reprogramming papers
The field is relatively new – there have only been around 19 papers so far on partial reprogramming. Even so, it can be a little confusing to navigate, so here are some things to know before we start doing a deep dive:
Not all partial reprogramming papers focus on rejuvenation – some studies only focus on therapeutic effects. This means that instead of performing partial reprogramming in old mice and seeing if they live longer or gain functions that they would have had when they were younger, these studies assess whether it helps mice recover from injury better. For the purpose of this article we will be excluding these studies in the summaries of rejuvenation effects — they are labeled “not rejuvenation” in Table 1 below.
Instead of using old normal mice, some studies use mice with progeria, a disorder that causes mice to age faster. In this disorder, mice have a genetic mutation that alters the structure of the nucleus, leading to disordered heterochromatin and DNA instability (two of nine hallmarks of aging). Progeria is not the same as aging but does capture some aspects of it. LAKI mice is a type of progeric mice model often used in partial reprogramming studies.
LAKI homozygous mice carry two copies of the mutated gene and live for 20 weeks on average.
LAKI heterozygous mice carry one copy of the mutated gene, develop the disease in a less severe way, and might be a closer representation of normal mice. They live for 35 weeks on average.
Wildtype mice live for 2.5 years on average (120 weeks)
In partial reprogramming studies, all LAKI mice are reprogrammable mice with 1 copy of OSKM.
Different studies do different things: some induce partial reprogramming in vivo in the whole body, some do in one part of the body, and some do it in vitro on certain types of cells. That’s why they also track different measurements in each paper. For ease of reference, below is a table that gives an overview of all 19 partial reprogramming studies and what effects they measure.
Table 1: 19 Partial reprogramming papers and what they measure
| Reference | Species | Induction location | Lifespan increase | Physiology | Molecular biomarkers | Not rejuvenation |
|---|---|---|---|---|---|---|
| Abad 2013 | Mice, reprogrammable | in vivo, whole body | Negative effects | x | ||
| Ohnishi 2014 | Mice, reprogrammable | in vivo, whole body | Negative effects | x | ||
| Manukyan 2014 | Human | in vitro, fibroblast | NA | - | HP1β mobility | |
| Seo 2016 | Mice, reprogrammable | in vivo, in situ brain | - | Rotarod tests Ladder walking test Neurogenesis Neuron survival & plasticity | - | x |
| Ocampo 2016 | Human, reprogrammable | in vitro, fibroblast | NA | - | Aging hallmarks | |
| Mice, reprogrammable | in vitro, fibroblast | NA | NA | Aging hallmarks | ||
| in vivo, whole body | - | Glucose tolerance Pancreatic islet size Differentiation capacity (satellite cells) | - | |||
| LAKI (homozygous) mice | in vivo, whole body | Median 33% Maximum 13% | External appearance Organ-specific deteriorations | Aging hallmarks | ||
| Guo 2017 | Mice, reprogrammable (club-cell-specific) | in vivo, club cells (lung) | - | Differentiation capacity | - | x |
| Doeser 2018 | Mice, reprogrammable | in vivo, on skin wound | - | Scar formation Fibrosis | Fibrotic marker genes | x |
| Gobel 2018 | Human | in vitro, mesenchymal stem cells | NA | Genetic abnormalities | No effects | |
| Olova 2018 | Human | in vitro, human fibroblasts | NA | - | Epigenetic clocks | |
| Sarkar 2020 | Human | in vitro, chondrocytes, endothelial cells, fibroblast | NA | Proliferation ATP production | Aging hallmarks Transcriptome Epigenetic clocks | |
| Mice | in vitro, muscle stem cells (both mice and human) implantation in mice | NA | Tetanic force Differentiation capacity Proliferation | - | ||
| Matellan 2020 | Mice, reprogrammable | in vivo, whole body | - | Memory index Neurogenesis | H3K9me3 | |
| Lu 2020 | Human | in vitro, neuron | NA | Neutrite area | - | |
| Mice | in vivo, whole body (safety only) and eye | - | Visual acuity RGC electrical activity Cell survival Axon regeneration | Transcriptome Epigenetic clocks | ||
| Gill 2021 | Human | in vitro, fibroblast | NA | - | H3K9me3 Transcriptome Methylome Epigenetic clocks | |
| Alle 2021 | Mice, reprogrammable | in vitro, fibroblast | NA | NA | Aging hallmarks | |
| in vivo, whole body | 16% third quartile no increase in median and max | - | - | |||
| LAKI (heterozygous) mice | in vivo, whole body | 31-33% median 13-25% max | Lean mass Grip strength External appearance Organ-specific fibrosis | Methylome | ||
| Roux 2021 | Mice, reprogrammable | in vitro, fibroblast, adipogenic cells, myogenic cells | NA | - | Transcriptome Transcriptomic clocks | |
| Chen 2021 | Mice, reprogrammable (cardiomyocyte-specific) | in vivo, cardiomyocytes | - | Heart function Proliferation | Transcriptome | |
| Chondronasiou 2022 | Mice, reprogrammable | in vivo, whole body | - | - | Transcriptome Methylome Metabolites | |
2. What is rejuvenation?
The first step to understanding how partial reprogramming rejuvenates cells is defining what rejuvenation means. No one agrees what aging truly means. By that extension, it is also hard to tell whether a treatment makes an animal younger or not. The most straightforward way to answer that question is to measure lifespan – does a treatment actually extend the lifespan of an animal?
However, lifespan extension might not be the be-all-end-all – a treatment might be promising even if all it does is make an old cell look like a young cell. But that gives us another question: what does it mean for an old cell to look like a young cell? There’s also no good way to answer that, so different studies measure different things: transcriptome, aging hallmarks, epigenetic clocks, etc. (as evidenced by Table 1).
As of now, the metrics most studies use to measure rejuvenation are: lifespan extension, physiological improvements, and molecular biomarkers improvements. We’ll go into an overview of each of the metric below.
2.1. Lifespan extension
This is how one measures lifespan extension:
Take a cohort of mice undergoing no treatment, and observe how many die at which age until the last one dies. Plot the Kaplan-Meier curve, where median lifespan is when 50% of the cohort dies, and maximum lifespan is when the last one in the cohort dies.
Now do the same thing for mice treated with partial reprogramming.
When you compare the curve for untreated condition and that of treated one, you can see how much a treatment extends the median and maximum lifespan.
Kaplan-Meier curve. The gray curve is the control, and the blue and red curves are treated mice. Image credit.
2.2. Physiological improvements
As we age, many things get worse. For example, muscle mass decreases, memory declines, etc. That’s why we care not only about lifespan extension, but also the physiological improvements that come with a treatment (i.e. healthspan). Physiological improvements measure whether the function of body parts, organs, or cells return to their youthful states and vary a lot depending on what you’re measuring. For example, from Table 1 we can see that a few of these metrics include:
External appearance (fur color, spine curvature, etc.)
Grip strength
Visual improvements
Memory improvements
Cell proliferation
2.3. Molecular biomarkers improvements
For certain types of cells (e.g. human cells, since we can't do risky treatments on live people), we cannot measure their lifespan or functional improvements. In this case, molecular biomarkers are the most appropriate metrics. Generally, they can be divided into three categories: specific aging biomarkers, transcriptome, methylome and epigenetic clocks.
Specific aging biomarkers
There are nine hallmarks of aging, which can be assessed using certain molecular signatures. We can measure the hallmarks of a cell to see if they have been restored to a younger level if we want to determine if a cell is getting younger.
However, measuring hallmarks has its problems:
There are no universal biomarkers for certain hallmarks.
Consider cellular senescence, a phenomenon in which cells enter a permanent cell-cycle arrest and cease to replicate. Currently, senescence is measured with a combination of different biomarkers: senescence-associated secretory phenotype (SASP), β-galactosidase activity, p16, p53, p21, and more. However, not all senescent cells have these biomarkers, and some non-senescent cells express biomarkers as well. As a result, quantifying the improvement of certain hallmarks can be tricky.
Not all hallmarks are measured in every study, due to different reasons not specified in the papers:
The hallmark is not measurable
There’s no change between young and old cells in that hallmark in a particular cell type
The hallmark was not improved with partial reprogramming, so it was not reported
We don’t know which hallmarks are more important than the others.
To what extent can we really say that a cell becomes younger because some hallmarks of aging are reversed? If a cell’s proteostasis improves, but its senescence does not decrease, would it be considered rejuvenated? Since not all hallmarks are measured, this makes comparing the extent of rejuvenation even more challenging.
Transcriptome
Another way to check whether a cell is getting younger is to look at all the gene expression in the cell, aka the transcriptome.
Studying the transcriptome generally consists of the following steps:
Obtain the transcriptomes of young and old cells
Obtain the transcriptome of old cells treated with partial reprogramming
Use statistical analysis to compare the transcriptomes of young, untreated old, and treated old cells to see how much the treatment “rejuvenates” the cell. These include:
Principal component analysis (PCA): this dimensionality reduction method allows us to see how the smaples cluster together. If the treated old cells are closer to the young cells than the untreated old cells, then that implies the treatment partially "rejuvenated" the aged genome in those samples.
PCAs from Chondronasiou 2022 and Sarkar 2020
Transcriptomic age calculation: some studies create their own aging score based on transcriptomes (Gill 2021, Roux 2021). They do this by various methods, including building a predictor based on a public data set or using a gene set scoring method.
Overall, transcriptome analysis is a little more standardized and systemic than looking at specific aging markers. However, it still has its own challenges, such as difficulty of interpreting PCAs.
Methylome and epigenetic clocks
DNA methylation is a type of epigenetic change. DNA methylation happens when you stick methyl groups to the DNA, which stops the gene from being transcribed. The methylome is the collection of all DNA methylation patterns in a cell.
Just like the transcriptome, the methylome also has specific aging patterns that can be analyzed using the same methods described above. Thus, some studies determine how much the methylome is rejuvenated by comparing the patterns across untreated old cells, treated old cells, and young cells. It has the same set of challenges as using transcriptome to determine rejuvenation.
Another way to determine whether a cell is rejuvenated is to calculate its epigenetic age. Most epigenetic age calculations involve using methylation sites as inputs to a predictor trained on chronological age and mortality. For partial reprogramming papers particularly:
In mice cells, we don’t see the use of epigenetic age very much. The only papers that calculates it are Lu 2020 and Browder 2022
In human cells, the most popular clocks are Horvath’s multi-tissue and skin-and-blood clocks
When we analyze transcriptomes and hallmarks, we can usually identify how the affected genes and proteins contribute to aging. In contrast, clock methylation sites are usually found in "silent genes," which do not change anything in a noticeable way. Therefore, one of the limitations of epigenetic clocks is that it’s difficult to interpret.
In addition, we don’t understand its causality. Does aging change the patterns of methylation sites in epigenetic clocks, or is it the other way around? If a treatment alters the epigenetic patterns, does it really mean that the cells are younger? As a consequence, like other molecular biomarkers, epigenetic clocks cannot serve as a sole indicator of a rejuvenated cell, even though their correlation with chronological age and mortality is significant.
3. partial reprogramming and rejuvenation
Table 2: Results of partial reprogramming in mice and humans. See here for a more detailed summary.
| Lifespan extension | Physiological improvements | Molecular improvements | Papers | |
|---|---|---|---|---|
| LAKI homozygous mice | • 33% median • 18% maximum | • Prevention of development of bradycardia (low heart rate) • Improved phenotypes in heart, skin, muscle, liver, stomach, kidney, spleen (became more similar to WT mice) • Restored proliferation in skin, stomach, kidney (partially up to level of WT mice) • No improvement in age-related weight loss | • Hallmarks of aging improvement in fibroblast (in vitro),liver, stomach, kidney, spleen, skeletal muscle, hair follicle • No effects on expression of Lamin A/C or Lamin B1 or accumulation of progerin | Ocampo 2016 |
| LAKI heterozygous mice | • 26% median • 16-18% maximum | • Significant reduction in age-related weight loss • Improved body composition (higher lean mass, lower fat mass) • Increased mean holding time and grip strength • Improved phenotypes in skin and bone • Reduced fibrosis in heart, lung, liver, kidney, spleen | • 640 genes are differentially expressed in fibroblasts (in vitro) compared to untreated mice – related to formation/regeneration of tissues and diseases like fibrosis, dilated cardiopathy, osteoarthritis • Differential methylation patterns in heart, lung, liver, kidney, spleen, skin compared to untreated mice. The best CpG signature is very specific to each organ | Alle 2021 |
| Wildtype mice | • No increase in median or maximum lifespan • 13% increase in third quartile lifespan | • Functional improvements in vision***, memory and object recognition*, glucose tolerance, and regenerative capacity of muscle* ** and heart*** • Improved phenotype in skin | • Hallmarks of aging improvement in fibroblast (in vitro),muscle stem cell, neuron. No change in senescence in liver and spleen • Rejuvenation of some metabolites in the serum • Transcriptomicrejuvenation in retinal ganglion cell***, skin, heart***, pancreas, spleen**, liver**. Transcriptomic rejuvenation (in vitro) in adipogenic and mesenchymal stem cells. No transcriptomic rejuvenation in muscle, lung, kidney • Methylomic rejuvenation in pancreas, liver, spleen, serum • 0.1-0.2 month decrease in epigenetic age in retinal ganglion cell***, skin, kidney. No change in epigenetic age in muscle, lung, liver, spleen | Ocampo 2016 Sarkar 2020 Matellan 2020 Lu 2020 Alle 2021 Roux 2021 Wang 2021 Chen 2021 Chondronasiou 2021 Browder 2021 |
| Human cells (in vitro) | NA | • Improved regenerative capacity of neurons* and muscle stem cells* | • Hallmarks of aging improvement in chondrocytes, endothelial cells, fibroblasts • Transcriptomic rejuvenation in fibroblasts • Decrease of 4.94 years in epigenetic age in endothelial cells. Decrease of 1.84 to 30 years in epigenetic age in fibroblast, depending on protocols | Ocampo 2016 Olova 2018 Lu 2020 Sarkar 2020 Gill 2021 |
| * denotes improvements in functions that might not be aging-related ** denotes conflicting results across different studies *** denotes targeted therapy | ||||
Overall, we see that partial reprogramming only induces lifespan extension on LAKI mice, with minor lifespan extension on normal mice. From this, one might argue that all partial reprogramming does is treat progeria instead of affecting aging. However, we know that is probably not true for two reasons:
First, Hutchinson-Gilford (a type of progeria) is caused by a genetic mutation that produces progerin, a mutant protein of lamin A, whose accumulation affects cells’ function and is a major cause of premature aging for LAKI mice. However, we see that partial reprogramming did not alter the expression of Lamin A or change the accumulation of progerin in LAKI homozygous mice. Thus, treated LAKI mice's longer lifespan is likely the result of amelioration of other aging processes besides progeria.
Second, a caveat for the above is that maybe the mutations produce a downstream impact which shortens lifespan in a way not found in physiologically-aged animals — so OSKM just addresses this "artificial" aging phenotype, and not true aging. However, we see that partial reprogramming does induce systemic improvements in aging-related biomarkers across various tissues in wild type mice as well as in human cells. Importantly, there are functional aging-related improvements (e.g. vision, heart function, glucose tolerance) in wild type mice, so it’s not just nebulous measurements of molecular biomarkers that are improved.
So why has lifespan extension not been seen in normal mice with this treatment? One reason argued here is that mice die primarily of cancer, and “lifespan extension in mice requires an intervention that addresses cancer directly or indirectly.” So even if partial reprogramming extends lifespan, if it doesn’t act on cancer, lifespan extension would not manifest in wildtype mice. Additionally, biology is complex – perhaps we have just not discovered the right protocol for this intervention yet. As we will see below, different dosages, protocols, and tissue types can result in different outcomes.
The question then is how promising is partial reprogramming actually? We know that it “rejuvenates” different tissues, but what is the extent of such rejuvenation? We still don’t know exactly, since there are no standardized biomarkers for aging. As explained in Section 2, different studies use various molecular measurements to estimate the extent of rejuvenation. Sometimes, the results (denoted with * in Table 2) don’t even agree with each other across different studies. Furthermore, we must distinguish between rejuvenation and therapeutic effects, since partial reprogramming can also induce positive effects that are unrelated to aging.
Distribution of partial reprogramming effects across different types of tissues. Y-axis denotes the number of studies.
Currently, skin and muscle stem cells are the two tissue types where partial reprogramming has demonstrated rejuvenation effects in both mice and humans, as well as being replicated in more than one study in the same species. Unsurprisingly, the most popular indication partial reprogramming companies are going after is dermatology, as we will see in Section 6.
In general, the findings summarized in the table above suggest that partial reprogramming is a promising rejuvenation intervention that can be applied to a variety of organs and tissues, but it is still in its infancy. We know that it targets a (likely universal) aging process, but we cannot conclude that the extent of rejuvenation it induces is higher than any other intervention. Below we will discuss the results in more detail.
3.1. LAKI homozygous mice
In 2016, Ocampo et al. came out with a seminal paper where partial reprogramming extended lifespan for the first time in mice, with a 33% increase in median lifespan and 18% in maximum lifespan. The researchers administered a cyclic protocol (1 mg/mL of dox for 2 days on, 5 days off) to progeric mice from 8 weeks old until death.
In addition to lifespan extension, the treatment also improved heart function. Normally, LAKI mice develop bradycardia, a condition where the heart rate is slow and the heart cannot pump enough oxygen-rich blood to the body. This is thought to be a major cause of their premature death. With partial reprogramming, LAKI mice’s heart rate improved significantly (although their heart rate still did not recover to the level of wildtype mice).
Physiologically, partial reprogramming induces phenotypic improvements in a variety of organs as well as improves their hallmarks of aging partially to the level of wildtype mice:
Skin: increase in epidermal and dermal thickness, decrease in keratinization, restored proliferation, improvement in 4 hallmarks (DNA damage, senescence, ROS, epigenetic alterations)
Muscle: partial restoration of stem cells
Liver: decreased senescence, decreased inflammation
Stomach: restored number of parietal cells and thickness of gastric epithelium, restored proliferation, restored H3K9me3, decreased inflammation
Kidney: decrease in tubular atrophy and interstitial volume, restored proliferation, restored H3K9me3, decreased inflammation
Spleen: rescue of macroscopic involution and lymphoid depletion of the white pulp, restored H3K9me3
Overall, partial reprogramming improved the physiology and hallmarks of aging across different organs in LAKI homozygous mice, but we don’t know which organs are more rejuvenated than others since not all hallmarks are measured in every organ (for example, inflammation was not measured in the spleen). In addition, a lot of the improvements are only partial restorations, i.e. they did not improve up to the level of wildtype mice. This makes sense as even with extended lifespan, treated LAKI mice did not live as long as wildtype mice.
3.2. LAKI heterozygous mice
As Ocampo et al. only studied the lifespan effects of partial reprogramming in LAKI homozygotes, Alle et al. extended this by studying LAKI heterozygotes. From their reasoning, LAKI heterozygotes are like an intermediate model between progeric and non-progeric mice since they are “very sensitive to improvements while not being so afflicted as to be difficult to interpret.”
There are two partial reprogramming protocols used in Alle 2021:
Cyclic protocol: 1 mg/mL of dox for 2 days on, 5 days off until death (Same as Ocampo 2016), started at 2 months old
Short protocol: 0.5 mg/mL of dox for 2.5 weeks at 2 months old
In the cyclic protocol, mice’s median lifespan increased by 26%, and maximum lifespan by 16%. In the short protocol, mice’s median lifespan did not increase, but their maximum lifespan did by 18%.
Alle et al. were interested in studying the health effects of the short protocol, so they also examined the physiological and molecular improvements it induced 5.5 months after treatment stopped:
Functional improvements in the muscles were observed, including improved hold times on rotarods and improved grip strength.
Physiologically, the short protocol improved mice’s body composition, lowering age-related loss of lean mass and fat accumulation. It also reduced fibrosis in heart, lung, liver, kidney, spleen, as well as increased skin’s dermal and epidermal thickness and bone’s volume of cartilage.
On a molecular level, they found that there were genes differentially expressed in treated mice related to the regeneration of tissues. They also discovered differential methylation patterns in heart, lung, liver, kidney, spleen, skin, in which the signature is very specific to each organ.
Unlike Ocampo 2016, this study did not compare the improvements with phenotypes in wildtype mice. Thus we only know that the improvements are in relation to untreated LAKI heterozygotes.
In this study, the most interesting finding was that partial reprogramming only needed to be done once when the mice were young to have the same maximum lifespan extension effect as doing it chronically throughout their lives. Additionally, we know that in Ocampo 2016, the aging hallmarks returned when induction stopped after 4 days, whereas in Alle 2021, the molecular changes stayed. There is, therefore, a time window in which partial reprogramming can have lasting effects on the cell, but before the cell becomes pluripotent.
3.3. Wildtype mice
There are 10 papers that studied the rejuvenation effects of partial reprogramming on wildtype mice. They each used different protocols that can be divided into three categories: short protocol (one-time OSKM induction), cyclic protocol (periodic OSKM induction over an extended period), and therapeutic protocol (local OSKM induction for therapeutic purposes).
Short protocol
Alle 2021 and Chondronasiou 2022 are two studies that used short protocol:
Alle et al. applied 0.5 mg/mL of dox for 2.5 weeks on 8-week-old mice
Chondronasiou et al. applied 0.2 mg/mL of dox for 1 week on 55- and 100-week-old mice
Alle 2021 is the only study that measured lifespan extension effect of partial reprogramming on wildtype mice. They saw that mice’s median and maximum lifespan did not increase, but their third quartile lifespan increased by 13%. They did not look at physiological and molecular biomarkers in these mice, but it would have been interesting to see if the lasting molecular effects 5.5 months after treatment seen in LAKI heterozygotes also applied to wildtype mice.
Chondronasiou et al. did not investigate lifespan or functional improvements, but rather the molecular rejuvenation of the short protocol. They looked at the mice’s pancreas, liver, spleen, and serum 2 weeks after stopping treatment:
The pancreas is said to be the most susceptible tissue to undergo reprogramming in reprogrammable mice. In this study, treated mice’s pancreas had 36% of methylation sites and 82% gene sets restored to youthful levels.
In the liver and spleen
5/15 mRNAs downregulated with age were restored with partial reprogramming, while senescence did not decrease with the treatment.
PCA of methylomes showed partial rejuvenation in the liver, not the spleen.
4/23 of serum metabolites were restored to young levels with partial reprogramming. Additionally, there was a partial reduction of Hsf4 methylation, which increases with age, in blood.
The rejuvenation effects did not seem as impressive as molecular rejuvenation seen in LAKI mice; however, this was done in very old mice whereas treatment in LAKI mice was started when they were middle aged. Additionally, the study did not examine mice’s physiology, so we don’t know if these molecular rejuvenation effects translate to any functional improvements. Nonetheless, these results indicate that there’s a possibility that partial reprogramming could work even when it is started late in life.
Cyclic protocol
Studies that used cyclic protocol (1 mg/mL of dox for 2 days on, 5 days off) include Ocampo 2016, Matellan 2020, and Browder 2022:
Ocampo et al. used a cyclic protocol for 3 weeks on 12-month-old mice
Matellan et al. used a cyclic protocol for 15 weeks on 6-month-old mice
Browder et al. used 3 types of cyclic protocol:
1-month cyclic protocol, started in 25-month-old mice
7-month cyclic protocol, started in 15-month-old mice
10-month cyclic protocol, started in 12-month-old mice
Ocampo et al. specifically looked at how partial reprogramming improved glucose tolerance and muscle injury recovery in old mice. After inducing OSKM for 3 weeks, they injured the mice’s beta cells (cells in the pancreas that synthesize and secrete insulin) and muscle, and observed how treated mice recover compared to young mice and old untreated mice. They found that treated mice had increased pancreatic islets (which contained beta cells) and showed higher glucose tolerance, indicating better pancreatic function. They also saw that treated mice had improved regenerative capacity and increased number of muscle stem cells compared to untreated mice after muscle injury.
Matellan et al. specifically looked at how partial reprogramming improved cognitive function in middle-aged mice. They found that treated mice had better memory index and object recognition abilities. However, this is not necessarily related to rejuvenation, since they did not evaluate young mice and compare their performance to middle-aged mice. They also saw that neurogenesis was not restored, only H3K9me3 (a hallmark of aging) was. Thus this finding of increase in cognitive function is a bit shaky, since the result was based on cognitive tests, which can be nebulous, without significant underlying molecular changes.
Browder et al. looked at how partial reprogramming rejuvenated the mice on a systemic level. Physiologically, they observed that skin proliferation was restored to the level of young mice in all of the protocols. To test the function of skin, they created wounds on 7-month-treated mice’s skin and observed that even though wound closure rate did not improve, there was more proliferation around the wound and reduction in fibrosis.
They also injured the muscle in mice treated with 7-month protocol and did not observe any changes in the regenerative potential. This is in contrast with the result from Ocampo 2016. This inconsistency might be due to the age at which partial reprogramming was induced – the mice in Ocampo 2016 were 12 months old, and the mice in this study were 15 months old. However, the difference in age is not very large, which made this inconsistency surprising.
On a molecular level, the results varied among protocols:
1 month protocol: no significant changes in the transcriptome and epigenetic age of the skin, kidney, spleen, liver, lung, and muscle
7-month and 10-month protocols:
Epigenetic age: decrease of 0.1-0.2 months in skin and kidney according to Lifespan Uber Correlation clock, but not in spleen, liver, lung, and muscle
Transcriptome: significant change in skin, but not in kidney, spleen, liver, lung, and muscle.
It is interesting to note that Chondronasiou 2022, which used a short protocol on much older mice, observed partial rejuvenation in the methylome for liver and spleen, even though the epigenetic clocks that Browder et al. used did not capture such rejuvenation. This highlighted how inconsistent measurements could lead to confusing results.
In addition, Browder et al. also performed metabolomic analysis on 10-month-treated mice. They saw that more than 9 major age-related metabolite changes in the serum were reversed.
Again, the rejuvenation effects on wildtype mice here seem underwhelming compared to results in LAKI mice: we only see some rejuvenation in skin, kidney, and metabolites. In addition, we also see that this study has some contradicting results to what was seen in Ocampo 2016 and Chondronasiou 2022, highlighting the need for more uniform biomarkers for rejuvenation.
Therapeutic protocol
Unlike studies in short and cyclic protocol, studies in therapeutic protocol looked at rejuvenation effects in specific tissues and organs instead of systemic rejuvenation. They include Lu 2020, Chen 2021, Sarkar 2020, and Wang 2021. They examined the rejuvenation effects of partial reprogramming on retinal ganglion cells, cardiomyocytes (heart cells), and muscle stem cells, respectively, with different protocols. In short, partial reprogramming was able to induce functional improvements in all of those cell types, which consist of:
Aging-related decline of vision and regenerative capacity of retinal ganglion cells (RGCs) after injury
Regenerative capacity of the heart after injury
Regenerative capacity of muscle stem cells after injury
This does not necessarily mean that all those cell types are “rejuvenated” with partial reprogramming. Only RGCs and cardiomyocytes exhibit rejuvenated molecular biomarkers. Muscle stem cells' ability to regenerate might not be a result of their rejuvenation, but of remodeling of the extrinsic niche involved in muscle regeneration independent from aging.
Eye – Lu 2020 (click to expand)
Lu 2020 was one of the two partial reprogramming studies that did not use reprogrammable mice. They also got rid of the c-Myc factor in OSKM and only used OSK to avoid oncogenic effects. AAV2 Tet-Off vectors were injected into the 11-month-old mice's eyes and OSK was expressed for 4 weeks.
In the study, they saw that treated old mice had improved visual acuity and electrical amplitude up until the level of young mice. There was no increase in the number of RGCs or axon density, so these visual improvements were not a result of cell proliferation. In addition, 90% of transcriptome in old mice’s RGCs was restored to young levels after treatment, and the epigenetic age, calculated with ribosomal DNA methylation clock, decreased by 3 months.
The same study also shows that when young and old mice had their optic nerve crushed, axon regeneration could be induced by partial reprogramming, either pre- or post-injury. The methylation patterns caused by the injury were also reversed with partial reprogramming.
Heart – Chen 2021 (click to expand)
Cardiomyocytes (CMs) are cells that drive heart contraction. In adult mice, CMs no longer undergo cell division, whereas fetal mice’s CMs have great regenerative potential because they can still proliferate.
Chen et al. used heart-specific-reprogrammable mice (where dox induction would only express OSKM in the heart) to test how partial reprogramming affects CMs. They induced 5 mg/mL of dox for 6 days on 4-month-old mice and found that it partly restored the proliferative ability of adult CMs. Treated adult CMs showed signs that they re-entered the cell cycle in vivo, but only underwent one round of division in vitro. Gene expression analysis showed that treated CMs’ profile resembled that of neonatal CMs.
The same study also looked at partial reprogramming as a therapeutic tool for injury. They induced partial reprogramming both before and after heart attack. All treatments showed decreased scar size, but pre-injury induction showed the most cardiac function improvement.
Muscle – Sarkar 2020 and Wang 2021 (click to expand)
Muscle is a popular tissue to look at in partial reprogramming studies. Recall that we have Browder 2022 and Ocampo 2016, which both induced whole-body OSKM in mice to see whether it helped them recover better after muscle injury.
Sarkar 2020 and Wang 2021 took a different approach by inducing OSKM locally. Sarkar et al. used an mRNA cocktail with OSKMNL on old mice muscle stem cells for 2 days, then transplanted them into old wildtype mice to see if they could exert the same force as young mice after muscle injury. Wang et al. induced OSKM specifically in the myofiber (a type of muscle tissue) of reprogrammable mice with a cyclic 1 mg/mL dox protocol for 3 weeks. Both found that their treatments improved the regenerative capacity of muscle stem cells after injury.
What's interesting about Wang 2021 is that when partial reprogramming was induced only in muscle stem cells, their regenerative capacity did not improve. This result indicates that muscle regeneration by partial reprogramming might not be due to intrinsic rejuvenation of muscle stem cells – instead, it might have been caused by remodeling of the extrinsic niche unrelated to aging. Therefore, positive effects in the muscle from partial reprogramming might not be good evidence for rejuvenation.
Overall, studies in therapeutic protocol show more promising evidence for rejuvenation effects in wildtype mice than short and cyclic protocols. It suggests that perhaps we haven't seen astonishing results in systemic studies with partial reprogramming in wildtype mice due to non-targeted delivery.
In vitro studies
We have covered most tissues with the aforementioned in vivo studies, but there remain two cell types that have only been studied in vitro: adipogenic and mesenchymal stem cells. Roux 2021 used lentiviral vectors to deliver inducible OSKM systems in these cells and induced them with 4 µg/mL of dox for 3 days on 3 days off.
This is the only partial reprogramming study using single-cell RNA-seq, so it provides a unique perspective on what is happening in the transcriptome. Youthful expression was restored in 3485/5984 genes in adipogenic cells, and in 712 genes in mesenchymal stem cells. What’s more interesting is that in mesenchymal stem cells, 4000 genes unrelated to aging were also changed with partial reprogramming. Because of this, partially reprogrammed old mesenchymal stem cells became more different from young cells compared to untreated old cells, even though they are considered to be rejuvenated.
According to these results, partial reprogramming has different effects on different cell types and also produces effects orthogonal to rejuvenation. Therefore, rejuvenation could just be a tiny byproduct of present partial reprogramming technology, and refining it, either by targeting delivery or by tailoring it to specific cell types, may yield a more significant result for rejuvenation.
3.4. Human cells
Partial reprogramming seems to have interesting rejuvenating effects on mice so far, but how applicable is it to humans? So far, the intervention has been used on five human cell types in vitro: muscle stem cells, neurons, chondrocytes, endothelial cells, and fibroblasts. Let's examine the results by cell type.
Muscle stem cells
Sarkar et al. used an mRNA cocktail with OSKMNL for 4 days on muscle stem cells from human donors in the 10-80 years old age group. They then transplanted the cells into old mice with injured muscle. They saw that partially reprogrammed stem cells from old humans were able to regenerate at the same rate as those from young humans.
However, as in the case of wildtype mice, improvement in the regenerative capacity of muscle stem cells might be caused by effects other than rejuvenation, so an improvement in regeneration can’t be definitive proof that cells are rejuvenated.
Neurons
Lu 2020 differentiated SH-SY5Y neuroblastoma cells into human neurons, then transduced them with OSK through AAV-DJs. They found that partially reprogrammed neurons were able to regrow their neutrite area 15-fold greater than untreated control 9 days after the axon injury. This is a great therapeutic use case, but we can’t say whether regeneration happened because of rejuvenating effects.
Chondrocytes
Osteoarthritis is an aging-related degenerative joint disease. It happens when chondrocytes, a type of cell in cartilage that cushions human bones, deteriorate.
In Sarkar 2020, chondrocytes taken from patients with osteoarthritis were partially reprogrammed with an mRNA cocktail of OSKMNL for 2-3 days. Partially reprogrammed cells showed improvements in osteoarthritis-specific phenotypes, including reduction in two hallmarks of aging (inflammation and mitochondrial ROS), as well as restored cell proliferation, ATP production, and increased antioxidants to the levels of young and healthy chondrocytes.
Endothelial cells
Sarkar et al. partially reprogrammed endothelial cells from humans aged 50-65 years old with an mRNA cocktail of OSKMNL for 4 days. They showed improvements in 6/9 aging hallmarks, including DNA damage, epigenetic dysregulations, senescence, inflammation, proteostasis, and mitochondrial dysregulation. However, they did not show any improvements in mitochondrial ROS, SIRT1, and telomere length.
Transcriptomically, treated old endothelial cells resembled young cells more than untreated old cells. Epigenetic age in treated endothelial cells also decreased by 4.94 and 1.62 years in age, according to Horvath pan-tissue and skin-and-blood clocks.
Fibroblast
Fibroblast is the only human cell type where several different partial reprogramming techniques have been used. Here we have an opportunity to compare the rejuvenation effects between different partial reprogramming techniques.
Ocampo 2016 used reprogrammable fibroblasts differentiated from human iPSCs. They induced dox for 4 days and saw that the fibroblasts showed improvements in 2 aging hallmarks (DNA damage and H3K9me3).
Olova 2018 applied Horvath’s multitissue epigenetic clock to a published reprogramming time course on human fibroblasts, where reprogramming was induced through OSKM with retroviral plasmids. The analysis showed that between day 11 and 15, the cells were partially reprogrammed and showed a drop of ~30 years in epigenetic age.
Sarkar 2020 transfected old human fibroblasts with OSKMNL mRNA cocktail for 4 days. They saw:
Improvement in 5/9 aging hallmarks (DNA damage, H3K9me3, proteostasis, ROS and mitochondrial potential, SIRT1)
Treated old fibroblasts transcriptomics resembled young cells more than untreated old cells
Epigenetic age in treated fibroblast decreased by 1.84 and 1.07 years in age, according to Horvath pan-tissue and skin-and-blood clocks
Gill 2020 induced OSKM through lentiviral delivery of inducible OSKM factors for 10-17 days:
One aging hallmark, H3K9me3, was measured and showed improvement.
The transcriptional age of treated fibroblasts decreased by 30 years
Epigenetic age in treated fibroblasts also decreased by 30 years in both Horvath’s multi-tissue and skin-and-blood clocks
The most dramatic reduction in epigenetic age was observed on human fibroblasts when partial reprogramming was applied for more than 10 days. The 4-day partial reprogramming protocol, on the other hand, had less of an effect on epigenetic age, but still showed rejuvenation through improvement in aging hallmarks and a rejuvenated transcriptome.
Overall, partial reprogramming's beneficial effects appear to extend to human cells as well, with therapeutic effects on human muscle stem cells and neurons, and rejuvenating effects on human chondrocytes, endothelial cells, and fibroblasts. Most of the protocols used on human cells have been fewer-than-4-day inductions. However, the most significant reduction in epigenetic age was obtained with partial reprogramming for more than 10 days on the fibroblasts. Thus it would be interesting to experiment with longer protocols for different human cell types.
3.5. Summary of rejuvenation effects
For LAKI mice, partial reprogramming is extremely encouraging. Besides lifespan extension, it also induces impressive improvements in aging-related physiological and molecular biomarkers across multiple organs such as the liver, stomach, kidney, muscle, heart, and lungs.
Nonetheless, without lifespan data in wildtype mice, how can we know how promising partial reprogramming is? Hence, most studies investigated the same question by measuring physiological improvements and molecular biomarkers to estimate the extent of rejuvenation in wildtype mice. Here is a summary of what we know so far:
Alle 2021 is the only study that measured lifespan effect in wildtype mice. They used the short protocol, which did not extend median or maximum lifespan, but did extend the third quartile lifespan by 13%. From the same paper, we know that the cyclic protocol, but not the short protocol, extended LAKI heterozygous mice’s median lifespan. Therefore, a low hanging fruit here is to apply the same cyclic protocol in wildtype mice.
In whole-body rejuvenation studies, molecular biomarkers in wildtype mice only showed modest improvements compared to LAKI mice. Particularly, Chondronasiou 2022 and Browder 2022 showed molecular improvements in serum, skin, kidney, and pancreas, but not in muscle, lung, liver*, or spleen*. There is a possibility that this is due to wildtype mice receiving the intervention at a much older age. Thus another low hanging fruit here is to experiment with inducing partial reprogramming in young wildtype mice.
Studies in therapeutic protocol (e.g. in the eye and heart) showed more promising evidence for rejuvenation effects in wildtype mice than whole-body studies. It suggests that perhaps we haven't seen astonishing results in systemic studies with partial reprogramming in wildtype mice due to non-targeted and non-universal delivery.
Partial reprogramming's beneficial effects extend to human cells as well. Particularly, impressive epigenetic age reduction was seen with a 10-15 day reprogramming protocol. However, data for human cells is still sparse. In light of this, it might be a good idea to experiment with a longer protocol for partially reprogramming human cells as well as wildtype mice.
Comparing rejuvenation effects among different studies also illustrates the need to standardize aging biomarkers, as there are a few conflicting results (denoted with *). Overall, partial reprogramming seems promising, and perhaps we have just not found the right protocol for it to manifest its full rejuvenating potential.
4. The mechanisms of partial reprogramming
4.1. Epigenetic remodeling
Partial reprogramming is sometimes called epigenetic reprogramming for a reason. During reprogramming, the epigenetic landscape of the cell is changed dramatically, and this might possibly contribute to rejuvenation. A few studies have confirmed this by interrupting epigenetic changes during partial reprogramming to see if the rejuvenation effect is diminished.
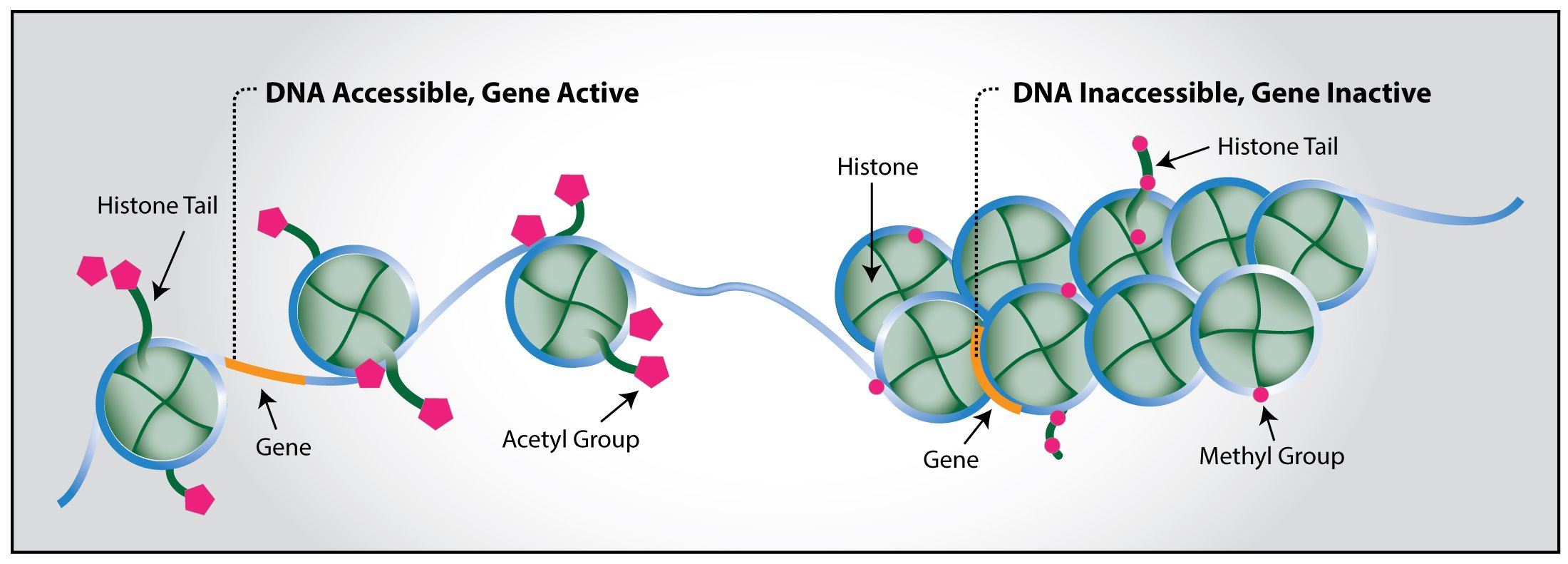
First, let's refresh our memory on epigenetics. Epigenetics consists of chemical modifications to the DNA that change gene expression without changing the DNA sequence itself. Modifications can occur on the DNA or on histones, proteins that bind to DNA. They include:
DNA methylation: adding a methyl group to the DNA strand. This typically silences gene
Histone methylation: adding a methyl group to a histone. This can either activates or silences gene
Histone acetylation: adding an acetyl group to a histone. This typically activates gene
Histone and DNA. Image credit.
The levels of these modifications either go up or down with aging, depending on cell types and species. We have seen that partial reprogramming induces epigenetic changes towards youthful states. If these changes are drivers of rejuvenation, blocking them should prevent rejuvenated phenotypes. Since they are chemical reactions that occur only in the presence of enzymes – methylation is catalyzed by methyltransferase, acetylation by acetyltransferase, demethylation by demethylase – one could use enzyme inhibitors to test this.
Let’s look at H3K9me3, the methylation of Histone 3 at location lysine 9. Generally, H3K9me3 declines with aging, and its drop is considered a biomarker for aging-associated epigenetic dysregulation. We have seen that the level of H3K9me3 is restored by partial reprogramming, particularly in mice fibroblasts (Ocampo 2016), mice hippocampus (Matellan 2020), and human fibroblasts (Sarkar 2020, Gill 2021). What would happen if we block the restoration of H3K9me3?
In LAKI homozygous mice fibroblasts, Ocampo et al. uses a H3K9me3 methyltransferase inhibitor during partial reprogramming and discover that this prevents the positive effects induced by the treatment, particularly the improvement of DNA damage and defects in nuclear envelope. In addition, a time course analysis shows that H3K9me3 restoration happens before the other two phenotypes.
We see the same thing with DNA demethylation. Knocking down Tet1 or Tet2, DNA demethylases, prevent the improvement in visual acuity and rDNA methylation age of old mice. It also prevents the axon regeneration in injured mice eyes and injured human neurons. Interestingly, increasing Tet1 expression alone without partial reprogramming does not help with axon regeneration.
Overall, this suggests that in partial reprogramming, epigenetic changes are required for rejuvenation but are not sufficient on their own.
4.2. Rejuvenation and dedifferentiation might be uncoupled
The biggest premise of partial reprogramming is that dedifferentiation and rejuvenation can be decoupled – that is, a cell can be rejuvenated with partial reprogramming without losing its identity. This relationship has been examined by a few studies.
Roux et al. conducted experiments where they induced partial reprogramming with different combinations of Yamanaka factors on mice mesenchymal stem cells and adipogenic cells. For each combination, they calculated:
An age score, based on the genes that were differentially expressed in the experiments and showed youthful gene expression restoration with partial reprogramming
An identity score, fit on semi-supervised, adversarial models using the Tabula Muris Senis
They found that age scores and identity scores were not well-correlated – some combinations show a significant reduction in age score without significant reduction in identity. For example, in mesenchymal stem cells (left image), SO has almost the same reduction in age score as OSKM without as much reduction in cell identity.
(As a side note, we know that OSK worked in mice RGCs and fibroblasts in Lu 2020, which also showed the same decrease in age score as OSKM in adipogenic cells here (right image). In mesenchymal stem cells (left image), we don’t see as much decrease in age score for OSK combination, likely due to low sample size)
Left: pooled screening of OSKM combinations on mesenchymal stem cells. Right: adipogenic cells. NT means “not treated”. Image credit.
In human fibroblasts, Olova et al. also found that age score and identity suppression are not well-correlated over a time course of reprogramming. They calculated age score with Horvath’s multitissue clock and identity with the expression of 19 commonly used fibroblast marker genes. Up to day 15, even though epigenetic age decreased dramatically, identity suppression was less dramatic.
Dynamics of epigenetic age and fibroblast identity expression over a 49-day reprogramming time course. Image credit.
So far, it seems that dedifferentiation and rejuvenation are not well-correlated, so there is a chance that we can find a way to decouple the two and turn partial reprogramming into a viable therapy. It is important to remember, however, that even though they did not have a linear correlation, they might have a nonlinear relationship, so it is not definitive that rejuvenation can occur without dedifferentiation.
4.3. Rejuvenation and re-differentiation
If rejuvenation and dedifferentiation can be uncoupled, what exactly rejuvenates the cells during partial reprogramming? Some might argue that it’s the process of redifferentiation – regaining cellular identities – that make the cells young again. A few studies have shown that during partial reprogramming, the cells move toward dedifferentiation, but return to their original states when OSKM induction is stopped. Let’s examine how these changes affect rejuvenation.
Roux et al. induced OSKM in mice adipogenic and mesenchymal stem cells for three days on, three days off. Through single cell RNA sequencing, they saw that the gene regulatory networks for cell identity were suppressed in these cells compared to untreated controls. However, RNA velocity (arrows in image below) predicts that these cells would eventually go back towards their control cell identities.
Partially reprogrammed mesenchymal stem cells are predicted to return to control cell identities in RNA velocity analysis. Image credit.
Gill et al. observed the same thing in human fibroblasts. They induced OSKM on these cells for 10, 13, 15, and 17 days, took cells that were successfully reprogrammed (called "partially reprogrammed intermediates"), and sequenced their methylome as well as Horvath's multitissue clock. They then left some of the “partially reprogrammed intermediates” to grow without doxycycline until day 51. These were “partially reprogrammed cells.”
They saw that the methylomes of “partially reprogrammed intermediates” (light pink) were moving towards states similar to those of iPSCs, whereas the methylomes of “partially reprogrammed cells” (dark pink) were clustered closer to those of control fibroblasts, implying that the cells dedifferentiate then regain their identities during the process.
Partially reprogrammed fibroblasts dedifferentiate then reacquire their initial cellular identity in their methylomes. Image credit.
To figure out where the cells “store” the memory of their identities, Gill et al. also looked at the methylomes of enhancers and promoters. They found that during complete reprogramming, fibroblast-specific enhancers were hypermethylated, but remained demethylated at the intermediate stages of transient reprogramming, suggesting that they were able to regain their identities through epigenetic memory in enhancers.
These results show that partially reprogrammed cells dedifferentiate then regain their identities in both transcriptomes and methylomes, and that the mechanism may come from epigenetic memory.
How does this affect rejuvenation? If transcriptomes and methylomes are changed during reprogramming then return to their original state, does that mean the cells regain the old phenotypes?
Not necessarily. Gill et al. measured the epigenetic age according to Horvath’s multitissue clock in negative controls, partially reprogrammed intermediates, and partially reprogrammed cells. From their results, we can see that the difference in epigenetic age between negative controls and partially reprogrammed intermediates vs partially reprogrammed cells was not significant for 10- and 13-day treatments. Interestingly, cells that were partially reprogrammed for 15 and 17 days showed the lowest decrease in epigenetic age. Gill et al. speculated that this might be because extended reprogramming makes reversion to original cell identity more difficult and results in cellular stresses that ‘re-age’ the methylome. This result suggests that the process of regaining identity in partial reprogramming might be important in rejuvenation.
Difference in epigenetic age between negative controls and treated cells. Chart made from data in Gill 2021.
Another result also implies the same thing. In a recent paper, Chondronasiou et al. induced OSKM for 1 week in wildtype mice and observed epigenetic rejuvenation in the pancreas. Notably, they saw that some epigenetic rejuvenation, specifically DNA demethylation, was not present at the end of the 1-week induction, but only appeared after 2 weeks of stopping OSKM. Thus, certain types of rejuvenation seem to only happen when the cell recovers after reprogramming is stopped, hinting that the redifferentiation process might be even necessary for rejuvenation.
Interestingly, in some cells such as neurons, loss of cell identity is involved in age-related neurodegeneration. It might be possible that with partial reprogramming, rejuvenation happens because of cells’ regaining of identity, not because of dedifferentiation. It will be interesting to test this by overexpressing identity genes in cells and see if they become rejuvenated.
5. Problems and limitations of partial reprogramming
5.1. Teratomas
Teratomas are a type of tumor that could be cancerous. The first two papers in partial reprogramming (Abad 2013, Ohnishi 2014) demonstrated that when too much OSKM is induced, mice will develop teratomas from dedifferentiated cells, resulting in weight loss and severe morbidity.
Different factors, including tissue and cell type, will influence the right dosage for partial reprogramming. For example:
In some cases, cell types may require dosages that would be harmful to the organism as a whole. In Chen 2021, mice were induced with OSKM only in the heart, using 5 mg/mL of dox for 6 days. This helps the mice recover faster after heart injury without any development of tumors. However, since mice can develop teratomas with 1 mg/mL dox induced for 1 week over the whole body (Abad 2013), inducing the amount of dox required for the heart over the whole body would be lethal.
Positive in vitro results may not necessarily translate to positive outcomes in vivo. In Lu 2020, fibroblasts from reprogrammable mice with two copies of OSK were given dox for 5 days in vitro. The cells showed no sign of Nanog (a marker of dedifferentiation) and displayed improved aging phenotypes. Conversely, in the same paper, when the same type of mice were given an equivalent amount of dox in vivo, they lost weight and died. Because all of the human data so far has been obtained in vitro, we will have to consider the possibility that the absence of tumors in those cells may not translate into clinical results.
On the other hand, we also do not know how much dedifferentiation is needed for teratomas to form. In Chen 2021, although partially reprogrammed mice showed signs of cardiac dedifferentiation, they were reversed when dox was removed, and the mice did not develop tumors over a two-month period. In Guo 2017, the cell identity marker for club cells (a type of lung cell) was also suppressed during partial reprogramming, but returned upon dox withdrawal, while mice showed no tumors nine months after induction. Therefore, even if partial reprogramming caused dedifferentiation in cells, at the right dosage it would not result in teratomas and cause long-term harm to organisms. These results suggest that we should be both cautious and optimistic about partial reprogramming since it is still quite nebulous in regards to the risks of teratomas.
5.2. Delivery
All in vivo partial reprogramming studies have been done in reprogrammable mice, with the exception of Lu 2020, which used AAVs to deliver OSK factors to wildtype non-programmable mice. At first, they tried to see whether their method could result in systemic rejuvenation. However, OSK expression was not enough for that to happen – it was initially observed in a few tissues in the study, but over time, dividing cells diluted it, and the expression was observed only in the liver with less than 20% rate. Thus Lu et al. switched their study focus to local rejuvenation in retinal ganglion cells instead.
Therefore, a current limitation of partial reprogramming is that we might not be able to rejuvenate certain tissues because we cannot deliver the factors there. Whole-body rejuvenation effects have been observed only in reprogrammable mice, which express the factors in almost every cell of their body. This cannot be applied to humans if we don’t have the means to deliver the factors to every cell. Reprogrammable mice are also 25 to 50 times more efficient in generating iPSCs than viral vectors, implying that partial reprogramming is also more efficient in reprogrammable mice. Moreover, different cell types might also require different dosages for rejuvenation, so we would need cell-specific delivery of factors.
Thus, to translate partial reprogramming with OSKM/OSK to humans, we would need cell-specific delivery methods for every cell.
5.3. Microenvironment
Reprogramming is not cell-autonomous, meaning that there are factors outside the cell, particularly its microenvironment, that would affect its success.
In vitro, reprogramming is influenced by inhibitory and supportive signals from the culture environment. For example, fibroblasts grown in serum-free media require more density than cells grown in serum-containing media. In vivo, another intriguing example is senescence. Senescent cells might be more difficult to reprogram, but they secrete senescence-associated secretory phenotype (SASP), which consists of cytokines and other senescence-associated molecules and facilitates reprogramming in neighboring cells.
We have seen the important role of microenvironment in a few partial reprogramming papers as well. In Chen 2021, in vivo reprogramming of adult mice’s heart produced iPSC-like clones within 21 days of treatment, whereas in vitro reprogramming of adult mice’s cardiomyocytes only went through one round of division and did not form iPSCs with 3 months of treatment. This suggests that there are factors in the heart’s microenvironment necessary for reprogramming of the heart cells. Furthermore, when mice’s partially reprogrammed heart was injured, proliferation was highest near the injury site, indicating that changes in the microenvironment after injury facilitate partial reprogramming.
Together, this implies that in addition to controlling for the dosage and delivery, we might also need to optimize the cells’ microenvironment in certain ways for successful therapeutic use of partial reprogramming.
5.4. Aging hallmarks that cannot be rejuvenated with partial reprogramming
Several aging hallmarks can be reset through partial reprogramming, but there are still a few things that cannot be corrected.
Nuclear and mitochondrial DNA mutations are one of them. We accumulate mutations as we age. In general, mutations are harmless, but some mutations are extremely harmful, such as those found in p53 genes, which make cells more prone to developing tumors. Generally, cells with these harmful mutations usually self-destruct, but as we age, our check and repair machinery gets worse, so these cells are allowed to survive and multiply. Additionally, some cells, such as heart and brain cells, do not divide (most of the time), so we cannot afford to let them self-destruct. Therefore, the most feasible way to fix these types of damage is to reverse the mutations, which partial reprogramming cannot do.
Metabolic aggregates in the cell and extracellular matrix are another type of damage that partial reprogramming might not be able to fix. Crosslinks and lipofuscins, which cause aging-related diseases such as macular degeneration, are examples of bad metabolic accumulations that we have not figured out how to clear. The extracellular matrix is particularly tricky because it is outside of the cell and doesn’t have an epigenome to reprogram. There is still a lot that we don’t know about the downstream effects of partial reprogramming though – it might be possible that it could induce specific gene programs to clear these damages on its own.
6. Alternative factors to OSKM
Since there are problems associated with using OSKM for partial reprogramming, some have pursued the path of finding alternative factors. These include:
Fewer combinations: Lu 2020 and Roux 2021 demonstrated that we do not need all 4 factors of OSKM for partial reprogramming to work. Particularly, Roux et al. showed that two factors (OS) might be just as effective as OSKM at reducing age score without suppressing identity as much. This opens the range of possibilities for different modalities, e.g. it’s easier to find small molecules that target two genes than four genes.
Multipotent factors: Roux 2021 also demonstrated that multipotent factors could be an alternative candidate to OSKM. They introduced Msx1, a factor known to play a role in limb and digit regeneration, in mice myogenic cells and saw that it reduced age score without dedifferentiating the cells. Testing other multipotent factors to see if they have the same effects would be a good idea.
Downstream factors: what about using downstream factors that are activated after OSKM reprogramming? Potentially, this could lead to identification of factors intrinsic to the OSKM process that drive rejuvenation without cell identity suppression. Wang 2021 identified Wnt4 as a downstream factor that drives muscle stem cells’ regenerative capacity after partial reprogramming (although they did not study rejuvenating effects of inducing Wnt4). As we see in Section 4.3, there’s also a possibility that regaining of identity is a major contributor to rejuvenation, so overexpressing identity genes is another interesting path to explore.
Germline rejuvenation: Kerepesi et al. uncovered a natural rejuvenation event during embryogenesis, evident by significant decrease in biological age. It would be very interesting to identify factors involved in such an event and see if they can be induced for rejuvenation.
Comprehensive screening: Finally, one could also identify new factors through comprehensive screening of all possible factors. For example, one could do a CRISPR screen over the genome, resulting in identification of 50 novel reprogramming factors. This is the approach taken by Shift and potentially other startups in the discovery category.
7. Reprogramming rejuvenation companies
Now that we understand the current state of research in partial reprogramming, let’s examine the commercial landscape of the field. Currently, there are 9 companies that work on rejuvenation through reprogramming. In addition, although not included in the below analysis, Genentech and Apollo Health Ventures might also be pursuing this field.
The criteria for inclusion of these companies are:
Focus on rejuvenation, not just regenerative medicine
Underlying technology is likely to be applicable to different organs/tissues/cell types OR the company itself has the intention to do so
Table 3: Companies working on cellular programming for rejuvenation
| Name | Founded | Funding | Indication | Modality | Approach | Refs |
|---|---|---|---|---|---|---|
| Calico | 2013 | 3.5B* | N/A | N/A | OSKM Alternatives | Roux 2022 |
| Shift Bioscience | 2017 | <10M | N/A | mRNA | Alternatives | |
| Rejuvenate Bio | 2017 | 17M* | Pet longevity | Gene therapy | OSK | Macip 2023 |
| Turn Biotechnologies | 2018 | <20M | Dermatology | mRNA | OSKMLN | Sarkar 2020 |
| Reverse Bioengineering | 2019 | 63M* | Dermatology | Small molecule | OSKM | |
| Iduna Therapeutics | 2020 | 206M* | Ophthamology | Gene therapy | OSK | Lu 2020 |
| YouthBio Therapeutics | 2020 | <10M | N/A | Gene therapy | OSKM Alternatives | |
| Retro Biosciences | 2021 | 180M* | Immunology | Cell therapy | Alternatives | |
| Altos Labs | 2021 | 3B | N/A | N/A | Alternatives | |
| NewLimit | 2021 | 105M | Ocular disease Immunosenescence | N/A | Alternatives | |
| Marble Therapeutics | 2022 | NA | Dermatology | Gene therapy | Alternatives | |
| * subsidiary / working on other programs besides reprogramming | ||||||
These companies have different initial indications and modalities, and in general, their approaches can be split into two categories:
Partial reprogramming using OSKM and related combinations, such as OSK only or OSKMLN (OSKM + Lin28 + Nanog)
Inducing rejuvenation with OSKM alternatives (e.g. targeting different gene combinations)
All of these companies are in preclinical stages, but those that adopt the first approach are farther along – most have done in vivo preclinical studies, whereas companies using OSKM alternatives are still in the discovery stage. Interestingly, out of the three companies closest to clinical stage, two are pursuing indications in dermatology.
A map of reprogramming companies’ progress and approach
We’ll look at individual companies more closely below.
Calico
Calico is a longevity mega-company founded in 2013 as a subsidiary of Alphabet. It is a clinical-stage company with indications in neurodegeneration and cancer, as well as R&D labs working on basic research.
The lab of Jacob Kimmel, one of Calico's principal investigators, recently published a preprint on partial programming. The paper found that certain subsets of OSKM (e.g. OS only) induced as much rejuvenation as OSKM without suppressing identity as much. They also discovered an alternative to OSKM: Msx1, a multipotent gene that rejuvenates cells.
Kimmel stated that Calico is currently not thinking about pursuing partial reprogramming clinically and is “primarily pursuing this work to explore fundamental questions about aging.” In addition, he also left Calico to join NewLimit as their Head of Research in March 2022. Therefore, it’s likely that Calico is not going to be a big player in partial reprogramming for now.
Shift Bioscience
Shift was founded in 2017 based on the doctoral research of its founder. Its initial goal was to create a small molecule drug that can lower the population of dysfunctional mitochondria.
The team created a single-cell transcriptomic clock based on the Tabula Muris Senis to measure the rejuvenating effects of their intervention. They realized that the clock they invented not only measures aging but also identifies candidate genes other than OSKM that can be used to rejuvenate cells. Based on this finding, the company pivoted to reprogramming in 2021.
According to a recent slide deck, Shift has optimized their rejuvenation gene candidates and is preparing to move from discovery phase to in vitro studies for validation. The company has yet to identify an indication to pursue; they plan to file patents for mRNA drugs targeting rejuvenating genes and license the intellectual property to pharma for disease-specific development.
Turn Biotechnologies
Turn Biotechnologies was founded in 2018 based on research from Stanford University. They use an mRNA cocktail consisting of OSKMNL factors for rejuvenation, and focus on optimizing the delivery to control the safety of the intervention. A publication related to the company is Sarkar 2020, where an mRNA cocktail of OSKMNL was used to rejuvenate human fibroblast, chondrocytes, and endothelial cells.
Turn’s secret sauce is ERA – an in-house platform for mRNA delivery customized for specific indications. Their platform optimizes the mRNA according to the target they are going after as well as the lipid deliveries, creating a product pipeline for proprietary drug formulations. From the website: “Turn’s ERA Platform enables us to carefully control the time, duration and dosage of transcription factors to optimize the mRNA cocktail for each indication. For example, we can add or subtract specific factors, depending on the benefit they provide with specific tissue.”
Turn’s lead indication is in dermatology, but it also has programs in ophthalmology, immunology, osteoarthritis and cartilage damage, and musculature. The team is preparing to enter in vivo safety and efficacy trials prior to proceeding to an FDA IND application, with the hope of going into Phase I in late 2022. More info about the company can be found in VitaDAO’s analysis.
Reverse Bioengineering (AgeX Therapeutics)
AgeX was founded in 2017 as a regenerative medicine company. In 2019, it spun out a subsidiary, Reverse Bioengineering, focused entirely on using partial reprogramming for induced tissue regeneration and various therapies.
The company uses the traditional OSKM factors for reprogramming. It has patented a way to detect when embryonic-fetal transition is reached during reprogramming – the point in which the cells regain regenerative capacity without being back towards pluripotency. It has also filed patents on multiple delivery methods including AAV, RNA/DNA, exosomes, and small molecules, but its drug currently in the pipeline, iTR 1574, is a small molecule cocktail.
From AgeX’s presentation in 2020 (slide 20), it seems that initially the company planned to go after heart failure as an indication for the partial reprogramming technology. However, in the most recent update (slide 23), the company has switched to going after application in dermatology, specifically for scarless wound repair. The company has shown results on skin and hair regeneration in mice in vivo, and is expecting more preclinical results for dermatology at the end of 2022.
Iduna Therapeutics (Life Biosciences)
Life Biosciences was founded in 2017 as a longevity biotech company that targets different hallmarks of aging. It spun out Iduna Therapeutics in 2020 based on research (Lu 2020) from Harvard University, which shows that partial reprogramming with OSK improves old mice’s vision and reverses injury- and glaucoma-induced vision loss.
The company licenses IP that covers a patented gene therapy system for OSK expression. According to the pipeline published on its website, Iduna is in the middle of preclinical study with no timeline on clinical trials yet, going after ophthalmology indications.
YouthBio Therapeutics
Youthereum Genetics was founded in 2017 to translate partial reprogramming. The company was then succeeded by YouthBio in 2020.
YouthBio is developing rejuvenation gene therapies in which partial reprogramming can be induced periodically through activation of OSKM with doxycycline (similar to how reprogrammable mice are partially reprogrammed in experiments), as well as alternatives to OSKM. The company plans to develop gene therapies for humans (unknown indications) as its next steps. It is at an early preclinical stage, building on in vitro results to in vivo studies.
Retro Biosciences
Retro was founded in early 2021. It is pursuing multiple programs, one of which includes cellular reprogramming approaches.
Retro’s launch announcement states that their reprogramming program is “closest to fundamental research” and that they are working toward clinical proof-of-concept over the next four years — where they will invest “heavily in single-cell multi-omics, machine-learning-based computational biology, and lab automation,” indicating their aim to find alternative factors to OSKM for rejuvenation. Their initial indication is likely in immunology, given their job posting for an immunologist in their partial reprogramming program.
Altos Labs
Altos Labs was founded in mid 2021 and has recruited an impressive lineup of big names in science with $3 billion in funding. It is the single largest amount of funding dedicated towards solely reprogramming for rejuvenation so far. The company coined a new term “rejuvenation programming” to describe their approach, indicating that it plans to find new factors for rejuvenation besides OSKM.
Morgan Levine, one of Altos’s principal investigators (PI), described the company’s organization structure as similar to academia: each PI has their own labs with postdocs and PhD students.
There will be a commercialization of research eventually, but PIs will still have intellectual freedom to pursue whatever interests them instead of being told what projects to work on. It seems that Altos is replicating the model of the early days of Bell Labs.
NewLimit
NewLimit was founded in late 2021. The company is in the discovery phase for finding alternative reprogramming factors, as evidenced by what is said on their website: “An example of the basic research we will be pursuing is mapping age dependent transcriptional and epigenetic shifts using single cell sRNA/ATACseq.” It is looking at ocular disease and immunosenescence as the first indications to pursue.
REjuvenate bio
Rejuvenate Bio was founded in 2017 with the goal of commercializing gene therapy for three longevity-associated genes, FGF21, sTGFβR2, Klotho. In January 2023, the company published a paper showing that a subset of Yamanaka factors (OSK) as sufficient to extend the remaining median lifespan of aged wild type mice by 109% (Macip et al. 2023). It looks like the company is planning to commercialize partial reprogramming with OSK as a therapeutic for dogs (unknown indication), as indicated by their animal health pipeline.
Marble therapeutics
Similar to Rejuvenate Bio, Marble Therapeutics was spun out of George Church’s lab. The company is currently in stealth mode without a website. From an interview with the CEO, the company seems to be in discovery phase for finding alternative reprogramming factors through human multi-omics data, starting with skin cells.
8. Closing thoughts
We went into this deep dive to understand how promising partial reprogramming is, and what is keeping it from being applied to humans. As a whole, it seems quite promising: it not only improves biomarkers across tissues, but also improves aging-related functions. Even though systemic in vivo experiments of wildtype mice only showed modest outcomes so far, it might be because the protocol has not been optimized. Low hanging fruit optimizations include starting the protocol at an earlier age as well as having a longer induction period. In addition, in vivo targeted treatment in wildtype mice demonstrated impressive rejuvenation effects, implying that non-targeted and non-universal delivery may have reduced the effectiveness of in vivo systemic studies. As a result, besides protocol optimization, technology advancement in adjacent areas like gene delivery would also be required in translating partial reprogramming.
Of course, the elephant in the room is cancer risk. We have seen that partial reprogramming inevitably dedifferentiates cells. However, dedifferentiation is not always bad – if administered at the right dosage, it is reversible and won't cause teratomas or damage to organisms. On the other hand, there are also additional efforts in finding alternative factors to OSKM that are safer and more effective, both in academia and industry. It's important to optimize what works (OSKM) and discover new factors for reprogramming rejuvenation. In this sense, it is a positive sign that half of the companies in reprogramming are focusing on optimization of OSKM and the other half are focusing on new discoveries.
Because aging is not considered a disease, it’s also important to think about what indications to pursue. Currently, skin and muscle stem cells are the two tissue types where partial reprogramming has demonstrated rejuvenation effects in both mice and humans, as well as being replicated in more than one study in the same species. Therefore, it makes sense that two out of three companies closest to clinical trials, Turn and Reverse, are pursuing dermatology indications. One might argue that given the cancer risk of partial reprogramming, it is risky to pursue indications largely for cosmetic use first. However, we have seen that skin cells are the most responsive to rejuvenation effects of reprogramming, so it’s possible to use very tiny doses, where cancer risk is close to zero, to achieve outsized effects. We could draw an analogy here to Botox – the first drug to be approved for cosmetic use by the FDA. It is a neurotoxin that causes botulism, a type of food poisoning that could cause paralysis and even death. In cosmetic uses, however, it is injected in such a small amount in targeted areas that it does not pose a risk – most serious effects were from therapeutic rather than cosmetic use. So it might not be a bad strategy to pursue dermatology indications first.
Another promising indication would be in immunotherapy, since approvals for cancer drugs are more risk tolerant than others. In immunotherapy, the patients’ immune cells (T cells) are engineered to fight against cancer. Over time, these cells might become exhausted and lose their effectiveness, giving cancer a chance to come back, a phenomenon called T cell exhaustion. Recent research showed that iPSC-derived T cells were able to sustain longer than normal T cells, thus, partially reprogrammed T cells might also be more effective at fighting cancer. As NewLimit is considering immunosenescence as an indication, this might also be on their roadmap.
Overall, we are still a long way from applying partial reprogramming to humans. Turn is the company closest to clinical trials in this space (expected to start late this year) – it may take a decade for partial reprogramming to be approved for therapeutic use. Even then, there are still many challenges that need to be addressed, such as creating both universal and cell-specific gene therapy, before this technology can be used for whole body rejuvenation. In addition, partial reprogramming cannot completely reverse all the signs of aging. So it is crucial that we continue to invest in the basic research of partial reprogramming and other areas of research, such as DNA damage repair, in order to make progress towards reversing aging. It's an exciting time for the field – let's keep on building!
In academic work, please cite this article as:
Nguyen, A. Partial reprogramming deep dive: the good, bad, and partially unresolved. March 2022. https://www.adanguyenx.com/blog/partial-reprogramming.
Many thanks to Henry Miller, Justin Chavez, Markus Strasser, Yuri Deigin, and Nathan Cheng for their thoughts on the draft. All remaining errors are mine.
Change logs
- 04/19/2023: add Rejuvenate Bio to section 7
- 05/04/2023: add Marble Therapeutics to section 7